Hongjian LI, Kwong-Sak LEUNG and Man-Hon WONG, Department of Computer Science and Engineering, CUHK
By using an innovative in-house toolset of in silico structure-based drug repositioning via ensemble molecular docking, researchers at Department of Computer Science and Engineering, collaborating with biochemists and medical doctors, have recently identified four candidate drugs that show anticancer activity against hepatocellular carcinoma in vitro and in vivo for the first time. These novel findings may represent alternative medications for the treatment of liver cancer once the repurposed drugs are put to clinical trials starting from phase II onward.
Hepatocellular carcinoma (HCC) is the most common type of liver cancer. Merely 30% to 40% of HCC patients are eligible for curative treatments, which include surgical resection as the first option, liver transplantation and percutaneous ablation. Nevertheless, tumor recurrence is frequently observed after surgical resection. Yet most HCCs become resistant to conventional chemotherapy and radiotherapy. Therefore the development of novel therapies against HCC has been heavily demanded.
The cause of HCC involves multiple pathways. The cyclin-dependent kinase (CDK) pathways have been established as important therapeutic targets for cancer treatment. CDKs are enzymes implicated in cell replication. Their role in tumor growth made them attractive targets long ago, but early industrial attempts at inhibiting CDKs to restore cell growth to normal level encountered toxicity issues. First-generation CDK inhibitors were not specific, inhibiting multiple CDKs and resulting in the type of toxicity and muted efficacy seen with old chemotherapies.
Among the many CDKs, CDK2 plays a pivotal role in regulating cell cycle transition and controlling cell proliferation. Hence, CDK2 inhibitors are effective anticancer agents. Although a number of CDK2 inhibitors have been reported in the literature and some have entered clinical trial phases, none of them has been approved for clinical use due to various reasons such as toxicity and low selectivity. Moreover, none of the described CDK2 inhibitors are for HCC treatment.
To quickly develop a novel therapy against HCC, researchers at the CSE department adopted the drug repositioning strategy, as finding new uses for existing drugs is much faster and cheaper with a lower attrition rate than discovering new drugs, which is exceedingly challenging and expensive. To this end, they collected and manually curated molecular data of 3167 approved drugs from USA, UK, Europe, Canada and Japan.
Structure-based virtual screening was then performed, using powerful in silico tools developed in house. These tools include a fast protein-ligand docking algorithm, a machine-learning scoring function, a general-purpose web platform, a hardware-accelerated molecular visualizer, and a de novo drug design program. "These tools are all free and open source," said Dr. Hongjian Li, the lead developer of the toolset, "we welcome everyone to use our tools and web server at http://istar.cse.cuhk.edu.hk, which have now been validated to be practically useful."
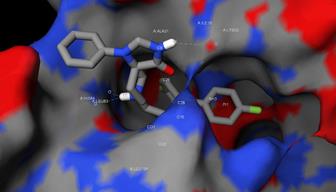
What is different from conventional structure-based virtual screening is that, in this project, as many as 44 holo structures of CDK2 bound with a ligand in complex were used to carry out ensemble docking. In this way, the protein flexibility induced by ligand binding was implicitly captured by utilizing multiple holo structures. The final score used to prioritize compounds was purposely designed to be the average score of that compound when docked to the 44 selected structures of CDK2 with their co-crystallized ligand deleted prior to docking. As a result, the top-scoring compounds would guarantee a consistently high binding strength on average across multiple target protein conformations.
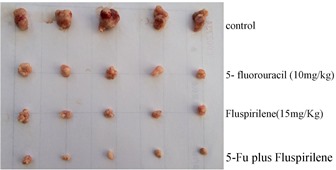
After ensemble docking followed by visual inspections of putative intermolecular interactions (Figure 1), more than 40 high-scoring compounds with previously unknown anticancer activity were purchased and examined via cytotoxicity assays in vitro. Those that exhibited submicromolar inhibitory effect to HCC cell lines HepG2 and Huh7 were further evaluated by cell cycle assays, western blotting and subsequently on nude mice in vivo (Figure 2). On day 21 after treatment, fluspirilene (15 mg/kg) resulted in a significant reduction of tumor weight and volume compared to the control (p<0.05), while making no significant change to the body weight.
Consequently, the FDA-approved antipsychotic drug fluspirilene was rediscovered as a potential CDK2 inhibitor and an anticancer agent for the first time. Our result suggests that fluspirilene, which has a long history of safe human use, could be the first CDK2 inhibitor for HCC therapy, as the majority of safety tests prior to phase II clinical trial could be possibly bypassed or easily passed again given the previous successful test results of the original indication. In addition, three other compounds identified in a similar way have been recently submitted for patent applications. Although clinical trials on humans are still to be conducted, these repositioned candidate drugs would have a high chance of providing alternative clinical therapies for the treatment of liver cancer.
This study was supported by grants from the Hsiang-fu Kung academician workstation of Kunming Medical University, National Natural Science Foundation of China (NSFC) 81272549, Key Lab project of Shenzhen (ZDSY20130329101130496) from China, the Direct Grant from the Chinese University of Hong Kong, the GRF Grant (Project References 414413, 772910 and 470911) from the Research Grants Council of Hong Kong.
For more details, please refer to the journal publication below.
Xi-Nan SHI, Hongjian LI, Hong YAO, Xu LIU, Ling LI, Kwong-Sak LEUNG, Hsiang-fu KUNG, Di LU, Man-Hon WONG, and Marie Chia-mi LIN. In silico Identification and in vitro and in vivo Validation of Anti-Psychotic Drug Fluspirilene as a Potential CDK2 Inhibitor and a Candidate Anti-Cancer Drug. PLoS ONE 2015;10(7):e0132072.
|
|
|
Figure 1: predicted binding conformation and intermolecular interactions of fluspirilene (ZINC ID: 00537755) in complex of CDK2 (PDB ID: 1GZ8).
|
|
Figure 2: in vivo effect of fluspirilene on the HCC tumor weight and volume in groups of five nude mice subcutaneously injected with Huh7 cells. This figure is modified from a published figure of the above-cited PLoS ONE journal paper, which is licensed under the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
|
|
|
