-
Chronic kidney disease (CKD) affects about 10% of adults worldwide. There are currently no specific treatments for kidney fibrosis, a major pathological hallmark of CKD.
-
A CUHK interdisciplinary research team has developed a self-therapeutic gold nanoparticle that can treat kidney fibrosis in mice.
Prolonged kidney fibrosis can lead to chronic kidney disease (CKD) and ultimately kidney failure, which requires dialysis or transplantation. A research team led by Professor Jonathan Choi Chung-hang, Associate Professor in the Department of Biomedical Engineering of The Chinese University of Hong Kong (CUHK)’s Faculty of Engineering, has developed a folic-acid conjugated gold nanoparticle that may offer a safe, effective treatment for kidney fibrosis. The research was recently published in the prestigious journal The Proceedings of the National Academy of Sciences (PNAS).
 (From left) Professor James Lau Yun-wong, Dr Cecilia Chan Ka-Wing, Professor Jonathan Choi Chung-hang, Professor Cheuk-Chun Szeto. (From left) Professor James Lau Yun-wong, Dr Cecilia Chan Ka-Wing, Professor Jonathan Choi Chung-hang, Professor Cheuk-Chun Szeto.
Currently, there are no specific treatments for kidney fibrosis, a major pathological hallmark of CKD. Medications are available to control blood pressure and reduce the rate of disease progression, but they are not entirely effective, and adverse effects such as hypotension and hyperkalemia are common. Nanomedicines hold great potential to treat CKD, but delivering them to renal tubules where fibrosis occurs is still challenging.
Bypassing the kidney delivery bottleneck with nanotechnology
Nanoparticles are promising for carrying drugs to the kidneys because the drugs’ delivery can be tailored by adjusting the nanoparticles’ physicochemical properties. Many existing kidney nanomedicines employ large nanoparticles (larger than 100 nm in diameter) to carry sufficient drugs and targeting ligands, but they need to disassemble unpredictably in the bloodstream to cross the glomerular filtration barrier (with a typical upper size limit smaller than 10 nm) before reaching the renal tubules where fibrosis occurs. Conversely, ultra-small nanoparticles (smaller than 5 nm) can pass through the glomerular filtration barrier, but they are often rapidly cleared from the body via urine, with limited retention in the kidneys for therapeutic purposes.
To bypass this bottleneck for delivery to the kidneys, Professor Choi’s team has invented a design to transport nanoparticles into the renal tubules of fibrotic kidneys. This ~7-nm particle can cross the glomerular filtration barrier without rapid urine clearance.
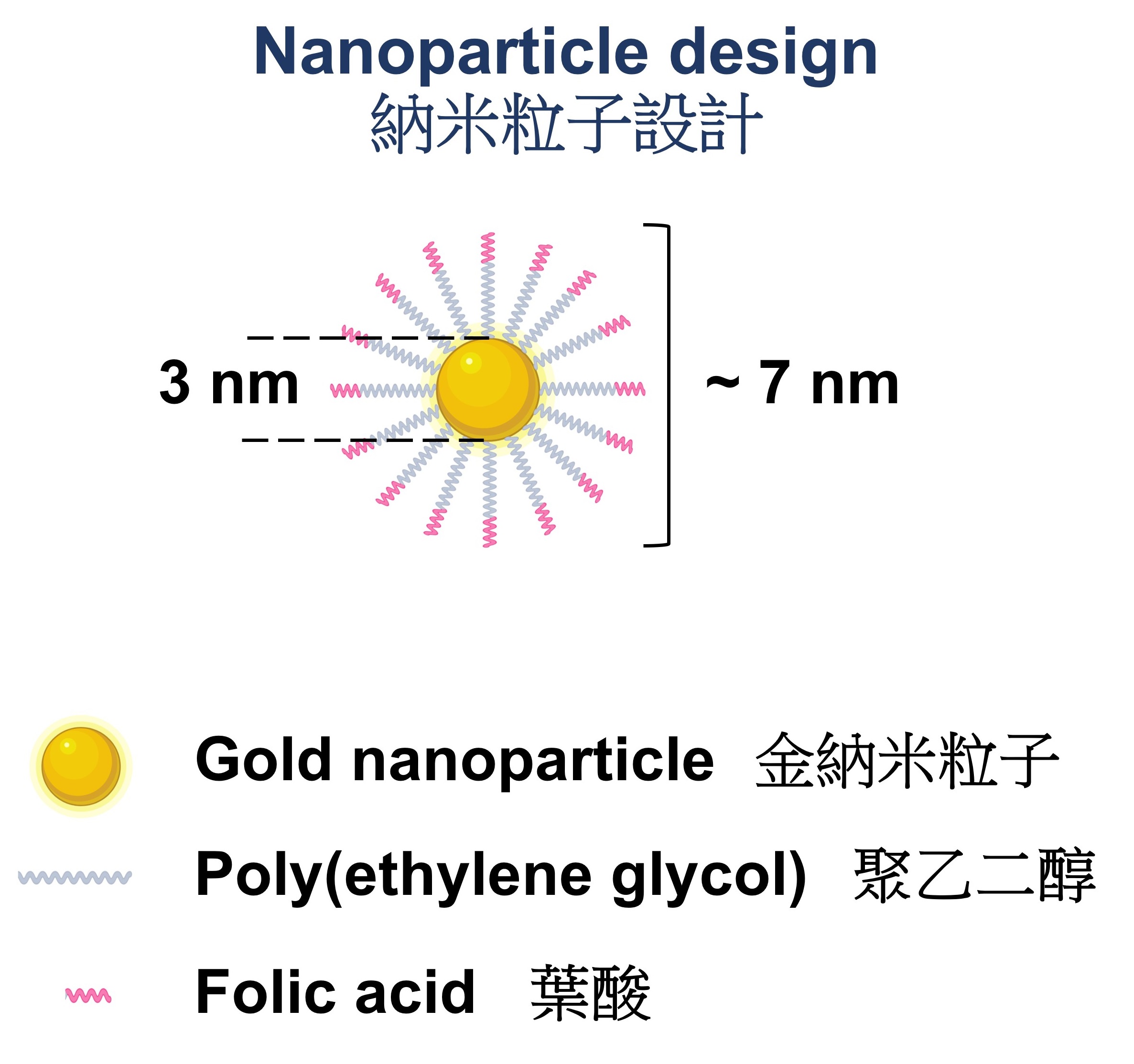 The folic acid-conjugated gold nanoparticle consists of a gold core of 3 nm in diameter and 32 strands of folic acid-poly(ethylene glycol). The entire structure has a diameter of approximately 7 nm. The folic acid-conjugated gold nanoparticle consists of a gold core of 3 nm in diameter and 32 strands of folic acid-poly(ethylene glycol). The entire structure has a diameter of approximately 7 nm.On the surface of a 3-nm gold nanoparticle, the researchers have attached about 32 molecules of folic acids, to form a folic-acid conjugated gold nanoparticle with a total size of around 7 nm. The conjugated folic acids can bind to the folate receptors on kidney tubules inside a fibrotic kidney, thus enhancing the intravenous delivery of the gold nanoparticles to the kidney. Professor Szeto Cheuk-chun of the Department of Medicine and Therapeutics at CUHK’s Faculty of Medicine (CU Medicine) said, “We discovered the localisation of folate receptors on some tubules in both mouse kidneys and biopsy samples from CKD patients.” Dr Cecilia Chan Ka-wing, the first author of the paper and a PhD graduate of CU Medicine’s Department of Surgery, added, “Our nanoparticles can preferentially accumulate in a fibrotic kidney, while leaving the other healthy kidney unaffected.”
Kidney fibrosis can be treated with a single injection of nanoparticles
Past CKD nanomedicines were mostly preventive. By contrast, the researchers have found that a single injection of folic acid-conjugated gold nanoparticles after the disease has become established was sufficient to reduce tissue degeneration, treat kidney fibrosis, and inhibit genes related to the extracellular matrix. Professor James Lau Yun-wong of CU Medicine’s Department of Surgery said, “This point is of clinical significance, given that early development of CKD is asymptomatic, which means patients are mostly diagnosed at a later stage.” The nanoparticles can be eventually cleared from the body via urine and faeces, and no pronounced toxicity was observed seven days after injection.
Professor Choi concluded, “Gold nanoparticles smaller than 10 nm are therapeutic for kidney fibrosis. They can inhibit p38α MAPK, an enzyme that contributes to the development of CKD, without the aid of any chemical or biological drugs. We hope to continue our collaboration with CU Medicine and to validate the safety and efficacy of gold nanoparticles in humans. Ultimately, we hope to offer a safe, effective gold nanomedicine for patients with kidney disease.”
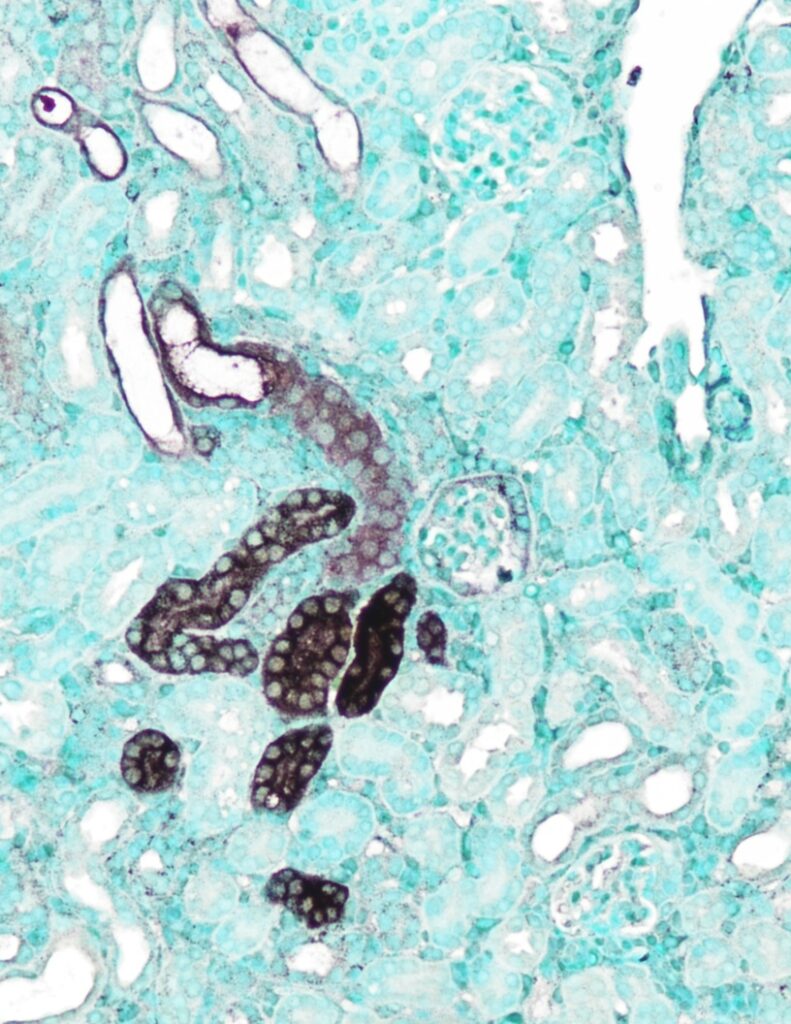 Sub-10-nm gold nanoparticles can cross the glomerular filtration barrier upon intravenous injection into mice with kidney fibrosis. When conjugated with folic acids, the gold nanoparticle can bind to the folate receptors on kidney tubules, enter tubules cells, and ultimately treat kidney fibrosis. In this kidney section, nuclei were stained with methyl green and gold nanoparticles were enhanced by silver staining. Sub-10-nm gold nanoparticles can cross the glomerular filtration barrier upon intravenous injection into mice with kidney fibrosis. When conjugated with folic acids, the gold nanoparticle can bind to the folate receptors on kidney tubules, enter tubules cells, and ultimately treat kidney fibrosis. In this kidney section, nuclei were stained with methyl green and gold nanoparticles were enhanced by silver staining.
|
